Kbr Lewis Dot Structure
You can use this example to find the electron dot diagram of hydrogen bromide HBr. LiF LiCl LiBr LiI LiAt.

A Truncated Version Of The Periodic Table Showing Lewis Dot Structures For The First 20 Elements Hydr Chemistry Lessons Chemistry Classroom Teaching Chemistry
The Lewis electron dot structures of a few molecules are illustrated in this subsection.
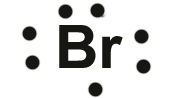
Kbr lewis dot structure. TeCl 2 SeCl 4 KrBr 2 Draw the Lewis structure Give the hybridization on the central atom TeCl 2 SeCl 4 KBr 2 sp 3 dsp 3 dsp 3. The central atom of this molecule is carbon. Till now you learned some common characteristics of this ionic salt.
Under standard conditions potassium bromide is a. Potassium a metal in Group 1 loses one electron to become a 1 ionBromine a non-metal in Group 17 gains one electron to become a -1 ionTogether they co. The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valance electron on each individual atom.
The Lewis Electron Dot Structures Concept Builder is shown in the iFrame below. Potassium bromide appears as odorless colorless crystals or white crystalline powder or white granular solid with a pungent bitter saline taste. Potassium bromide K Br is a salt widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries with over-the-counter use extending to 1975 in the US.
Non-valence electrons are not represented in Lewis structures. Oxygen contains 6 valence electrons which form 2 lone pairs. RbF RbCl RbBr RbI RbAt.
In order to draw an atoms electron dot diagram you need to know two things the atoms chemical symbol the number of. Potassium Bromide Kbr Structure Molecular Mass Properties Uses. NaF NaCl NaBr NaI NaAt.
For kbr we have an ionic compound and we need to take that into account when we potassium a metal in group 1 loses one electron to become a 1 ion bromine a non metal in group 17 gains one electron to. Since it is bonded to only one carbon atom it. The Lewis dot diagram is a table used for the elements and it shows you how many valence electrons there are.
For the following molecules. What is the Lewis structure of Cl2. KF KCl KBr KI KAt.
BONDING Types of Bonding Covalent Bonding Nonmetal with nonmetal Ionic Bonding Metal with nonmetal Metallic Bonding Metal with metal Lewis Electron Dot cont Step 1 Step 2 Step 3 Step 4 Step 5 Step 6 Step 7 Step 8 Steps to Write a Lewis Dot Structure Sum up all valence electrons of all atoms. Tim Manager Training Systems Solutions Opportunity Awaits. Give the orientation TeCl 2 SeCl 4 KBr 2 Tetrahedral Trigonal bipyramid Trigonal bipyramid Give the shape geometry.
For each one determine. Start with the Lewis Dot structure and then draw the corrected VSEPR Structure. Im not really sure if youre interested in the electron dot diagram of the potassium and bromine atoms or of potassium bromide KBr so Ill show you both.
FrF FrCl FrBr FrI FrAt. So Hydrogen is having 1 valance electron. For example when two chlorine atoms each with 7 valence electrons come together to form a diatomic chlorine molecule the Lewis structure shows that there will be a sharing of two electrons between the two chlorine atoms which allows both chlorine to be surrounded by 8 electronsLewis Dot Structures.
This way it will be easy to understand the reaction between potassium cation and bromine anion here is the Lewis dot structure of KBr-Image will be uploaded soon. What Im saying is that you cant write a Lewis dot structure for BrF2 by the normal method. To effectively integrate new employees during a pandemic demonstrates the excellent communication ongoing care and management skills of my colleagues and KBR leaders.
Potassium bromide is used as a veterinary drug as an antiepileptic medication for dogs. Arrange the Substances with polar bonds in order of increasing bond polarity. Are the bonds in each of the following ionic non-polar covalent or polar covalent.
Oxygen atoms have 6 valence electrons. KBR offered me a unique opportunity when the world was turned upside down. Lewis Structure of CO2.
It a step by step explanation of how to draw the kbr lewis dot structure. Clz g e. CsF CsCl CsBr CsI CsAt.
Now let us move forward to know the other properties of potassium bromide. The ClO2 Lewis structure has 19 valence electrons meaning that there will be an odd number of valence electrons in the structure. Its action is due to the bromide ion sodium bromide is equally effective.
NTP 1992 CAMEO Chemicals. Heres how that would look. Aqueous solutions are neutral pH about 7.
Potassium bromide is a metal bromide salt with a K counterion.
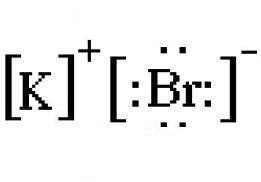
How Would You Represent Potassium And Bromine Using An Electron Dot Diagram How About Kbr Socratic
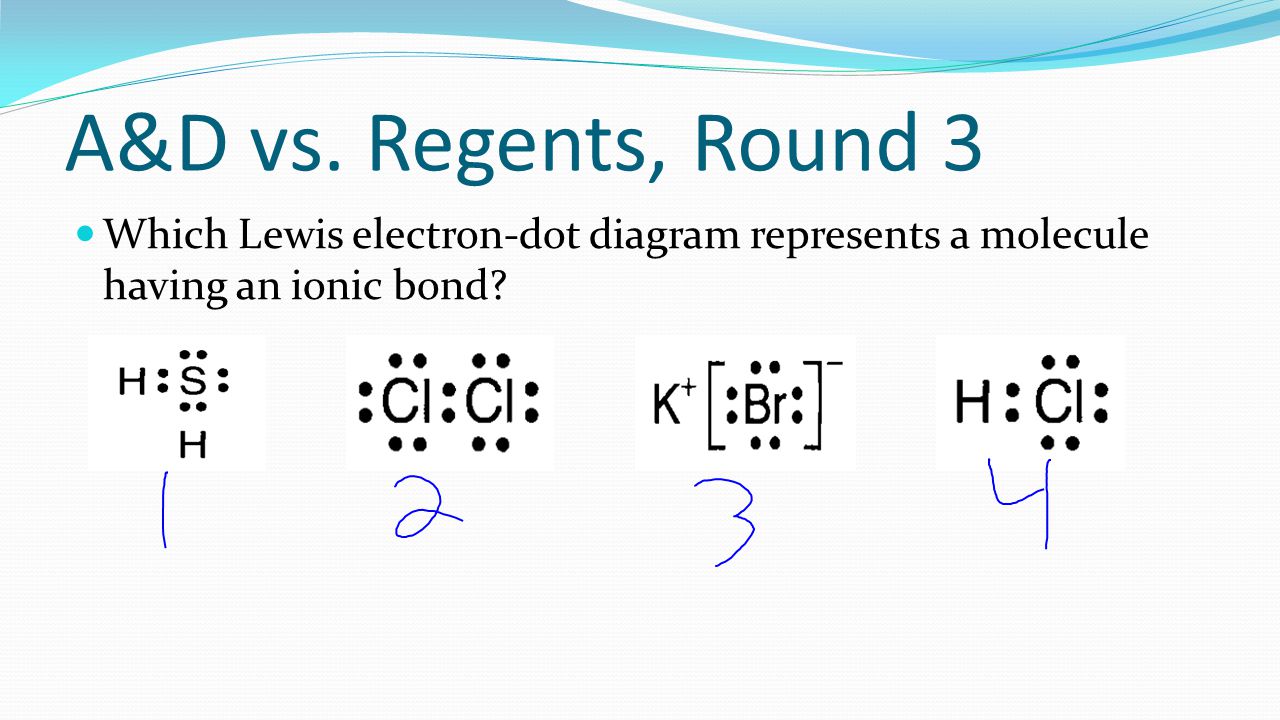
Do Now Which Substance Contains Bonds That Involved The Transfer Of Electrons From One Atom To Another Co2 Nh3 Kbr Cl2 When Sodium And Fluorine Combine Ppt Video Online Download

Add Electron Dots And Charges As Necessary To Show The En Ya Guru
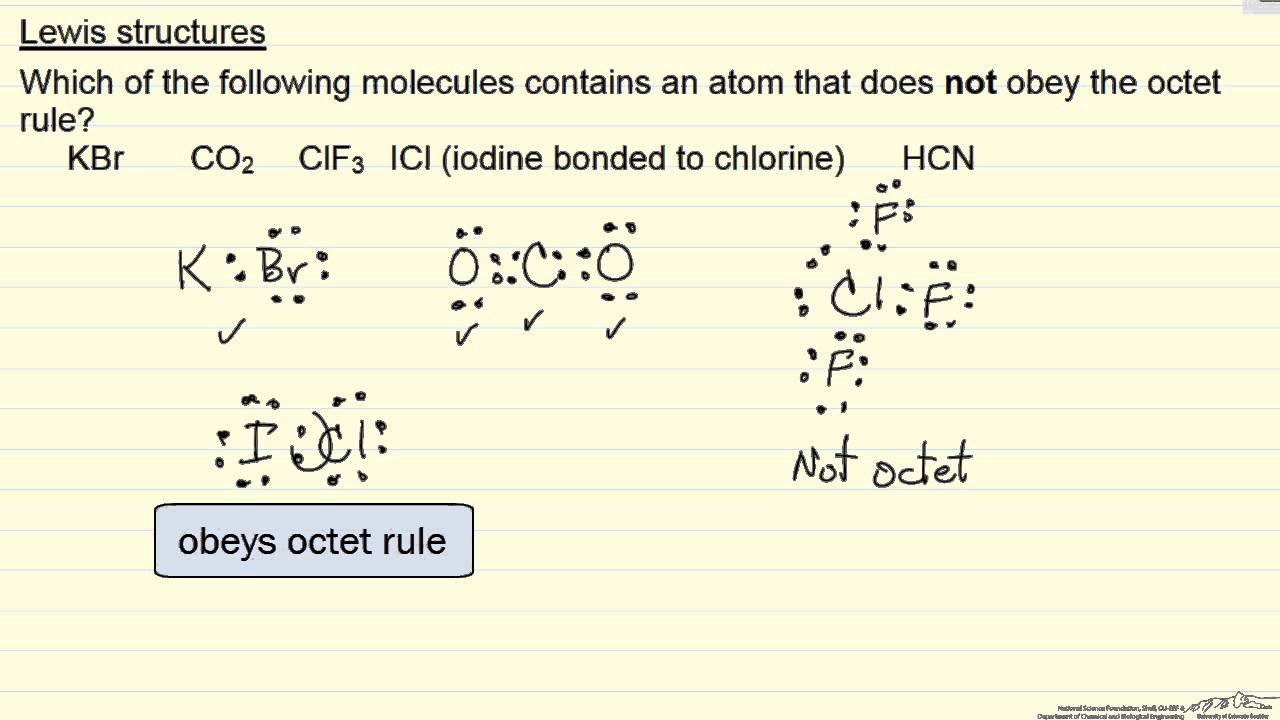
Lewis Structures Octet Rule Example Youtube

How Would You Represent Potassium And Brom Clutch Prep

Draw The Lewis Structure Of Kbr Potassium Bromide Youtube
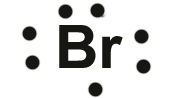
How Would You Represent Potassium And Bromine Using An Electron Dot Diagram How About Kbr Socratic
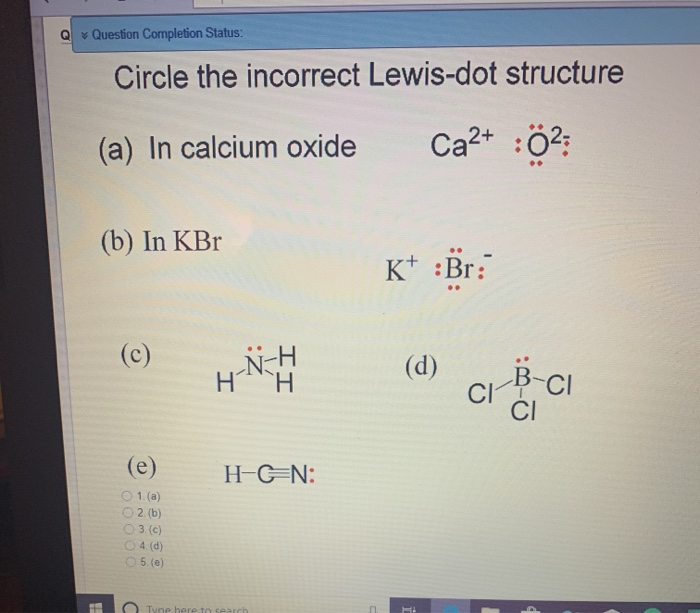
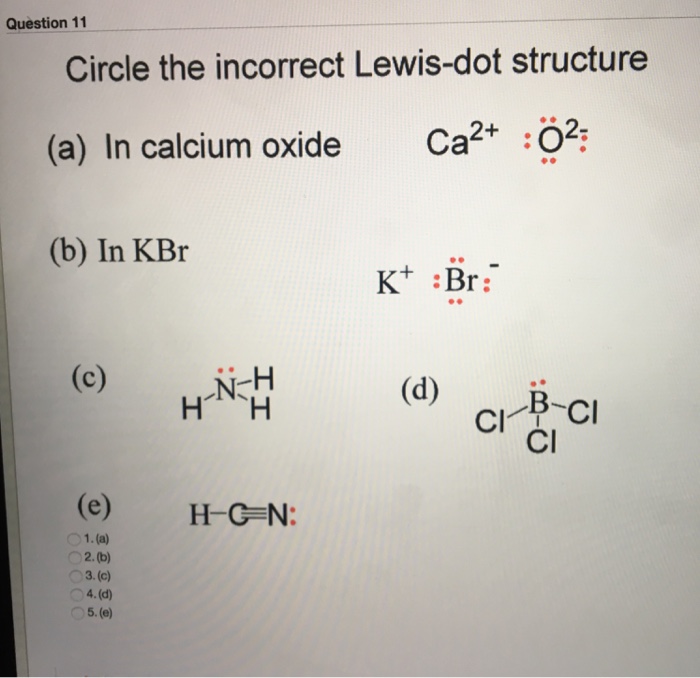
Q Question Completion Status Circle The Incorrect Chegg Com
How To Draw The Lewis Dot Structure For Ki Potassium Iodide Youtube

Born Haber Cycle For Kbr Youtube

How To Draw The Lewis Dot Structure For Ki Potassium Iodide Youtube
Question 11 Circle The Incorrect Lewis Dot Structure Chegg Com
E8f78393 Potassium Bromide 500g Findel International

How To Draw The Lewis Structure For Alcl3 Aluminum Chloride Youtube

How To Draw The Lewis Dot Structure For Kbr Potassium Bromide Youtube
Video Alcl3 Electron Dot Structure

How Would You Represent Potassium And Brom Clutch Prep
How Would You Represent Potassium And Bromine Using An Electron Dot Diagram How About Kbr

Potassium Fluoride Facts Formula Properties Uses Safety Data






Posting Komentar untuk "Kbr Lewis Dot Structure"